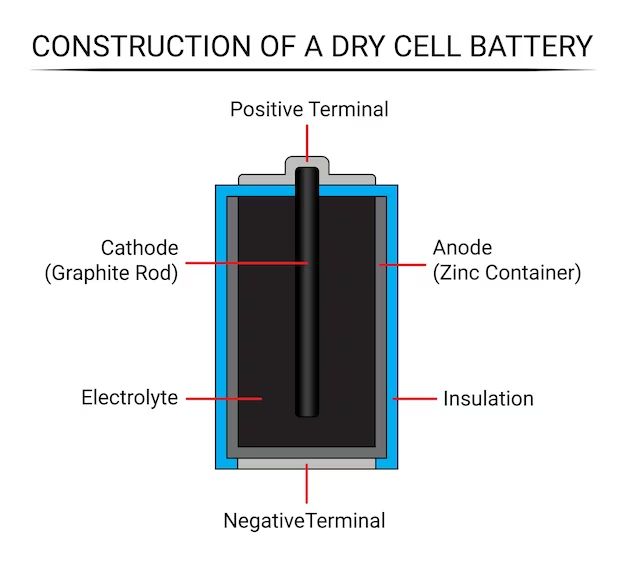
Batteries are electrochemical devices that convert stored chemical energy into electrical energy. They consist of one or more voltaic cells, each containing a positive terminal, or cathode, and a negative terminal, or anode. The terminals are the electrical contacts that allow current to flow out of the battery to power devices.
The terms cathode and anode refer specifically to the terminals within each voltaic cell that comprise the battery. The cathode is the positive terminal that collects electrons and is connected to the positive pole of the battery. The anode is the negative terminal that supplies electrons and is connected to the negative pole of the battery.
Page Contents
Cathode
The cathode is the positive terminal of a voltaic cell. It is the electrode where reduction reactions occur, meaning it receives electrons from the external circuit to balance the internal reaction in the cell. The cathode is connected to the positive pole of the battery.
Some key facts about the cathode:
- Made of a material that readily accepts electrons such as carbon or a metal like iron, zinc, or copper
- Attracts cations, which are positively charged ions
- In a galvanic or voltaic cell, the cathode is where the reduction reaction occurs
- The cathode has a positive polarity
- Connected to the positive terminal of the battery
The material used for the cathode depends on the type of battery technology:
- Carbon cathodes are used in zinc-carbon batteries
- Manganese dioxide cathodes are used in alkaline batteries
- Silver oxide cathodes are used in silver oxide batteries
- Mercuric oxide cathodes are used in mercury batteries
- Lead dioxide cathodes are used in lead-acid batteries
- Lithium cobalt oxide cathodes are used in lithium-ion batteries
During discharge of a battery when it provides electrical energy, the cathode is where the electrons enter after traveling through the external circuit. The cathode reaction in a zinc-carbon battery is:
MnO2 + H2O + e- → MnO(OH) + OH-
Here the manganese dioxide cathode accepts electrons and is reduced to manganese hydroxide. This allows ions to move freely between the cathode and anode internally, generating an electric current externally.
Anode
The anode is the negative terminal of a voltaic cell. It is the electrode where oxidation reactions occur, meaning it gives up electrons to the external circuit to balance the reaction in the cell. The anode is connected to the negative pole of the battery.
Some key facts about the anode:
- Made of a material that readily gives up electrons such as zinc, aluminum, or copper
- Releases anions, which are negatively charged ions
- In a galvanic or voltaic cell, the anode is where the oxidation reaction occurs
- The anode has a negative polarity
- Connected to the negative terminal of the battery
Common anode materials include:
- Zinc anodes used in zinc-carbon and alkaline batteries
- Lithium anodes used in lithium batteries
- Magnesium anodes used in magnesium batteries
- Aluminum anodes used in aluminum-air batteries
During discharge, the anode reaction in a zinc-carbon battery is:
Zn(s) → Zn2+ + 2e-
Here the zinc anode is oxidized to zinc ions and releases electrons to the external circuit. This creates a flow of electrons from the anode to the cathode within the battery, generating an electrical current.
Terminal Voltages
The voltage potential of a battery comes from the difference in electrical potential energy between its anode and cathode terminals. The cathode has a more positive electrical potential than the anode. It is this difference in potential that causes electrons to flow through the external circuit when the circuit is closed.
Some typical terminal voltages for common battery types:
- Zinc-carbon batteries: 1.5V
- Alkaline batteries: 1.5V
- Lithium coin batteries: 3V
- 9V PP3 batteries: 9V
- AA nickel-metal hydride batteries: 1.2V
- AAA nickel-zinc batteries: 1.6V
- 9V lithium-ion batteries: 9V
- Lead-acid car batteries: 2.1V per cell
Higher voltage batteries can be constructed by combining multiple cells together in series. The nominal voltage of the battery pack will be the sum of the individual cell voltages. For example, a lithium-ion battery pack made of 4 cells with 3.7V each will have a nominal voltage of 14.8V.
Battery Construction
Batteries contain one or more electrochemical cells connected in series or parallel inside a housing. Each cell contains an anode, a cathode, electrolyte, and a separator:
- The anode gives up electrons to the external circuit and is oxidized during the chemical reaction
- The cathode accepts electrons from the external circuit and is reduced
- The electrolyte allows ion flow between the anode and cathode to balance the electron flow
- The separator blocks physical contact between the anode and cathode but allows ion flow
In addition, batteries have the external positive and negative terminals for connecting to devices. These terminals are electrically linked to the anodes and cathodes of the individual cells inside.
Some batteries only have one cell. Examples include AAA, AA, C, D, and 9V consumer batteries. Other batteries connect multiple cells together:
- 2 cells connected in series: 9V PP3 battery
- 6 cells connected in series: 12V lead-acid car battery
- 3 cells connected in series: 3.7V lithium-ion battery pack
- 2 parallel sets of 2 cells in series: 4.8V pack of 2 lithium-ion cells
Having multiple cells allows higher voltages and currents to be achieved. The positive terminal of one cell is connected to the negative of the next to add their voltages together. Parallel sets add their currents together while keeping the voltage the same.
Battery Terminal Shapes and Sizes
Battery terminals come in different shapes, sizes, and orientations depending on the battery type and application:
- Small cylindrical batteries like AAA, AA, C, and D have terminals at either end of the cylinder.
- 9V PP3 batteries have snap type connectors on the top.
- Lithium coin cells have terminals on opposite flat sides.
- Lantern batteries have large threaded terminals on top.
- Lead-acid batteries have large tapered cylindrical terminals.
- Lithium polymer batteries have wire leads or flat contact pads.
- Cylindrical lithium-ion cells have a positive button terminal and a flat negative terminal.
Common terminal shapes include:
- Flat contact pads
- Rounded or pointed tips
- Raised buttons
- Threaded studs
- Spring loaded clips
- Rigid cylindrical shafts
- Flexible wire leads
Larger high capacity batteries may have even more complex arrangements and geometries for the terminals and interconnects between cells.
The size of the terminals must be made sufficiently large to handle the expected electrical current without overheating. They are also designed to make secure mechanical and electrical contact with battery holders or device connections.
Battery Polarities
Battery terminals have a defined polarity based on whether they are connected to the cathode or anode:
- The positive terminal is connected to the cathode.
- The negative terminal is connected to the anode.
This allows current to flow properly from the anode to the cathode through the external circuit when the battery is connected correctly. The positive terminal has a higher electrical potential than the negative.
Many battery types are designed so that it is impossible to install them backwards:
- AA and AAA batteries have the positive (cathode) terminal on the smaller end.
- 9V PP3 snap connectors only fit together one way.
- Lithium coin cells only insert with the correct polarity.
However, it is still important to pay attention to polarity when installing batteries, especially when mixing different battery chemistries which may have conflicting standards. Reversing the polarity forcibly charges the battery in reverse, which can damage it or cause leakage or rupture. The device being powered can also be damaged.
Some tips for getting the correct polarity:
- Look for + and – markings near the terminals
- The longer, more pointed terminal is often positive
- Colored wires or markings may indicate polarity
- Consult reference manuals for technical specifications
- Use a voltmeter to double check if uncertain
Maintaining the proper polarity when connecting and installing batteries is essential for safe and correct operation.
Battery Terminal Materials
The terminals of a battery are the electrical contact points and must be made of materials that conduct electricity well. They also need to be resistant to corrosion and chemical reactions with the electrolyte used within the cells. Common materials include:
- Steel or iron – Used on conventional batteries like alkaline and zinc-carbon. Can be nickel-plated to resist corrosion.
- Brass – Brass terminals provide excellent conductivity and are non-sparking for safety.
- Copper – Pure copper has high conductivity but can corrode. Often plated with nickel.
- Aluminum – Lightweight and conductive but oxidation is a problem so terminals are treated.
- Lead – Used for lead-acid battery terminals. Can be alloyed with other metals.
- Gold – Gold plated terminals provide maximum conductivity and corrosion resistance.
- Nickel – Nickel plating prevents corrosion on steel or copper terminals.
Factors for choosing terminal materials:
- High electrical conductivity to minimize resistance
- Mechanical strength for durability
- Heat resistance since currents can cause heating
- Resistance to chemical reactions with electrolytes
- Minimal tendency for sparking or arcing through air
- Low cost for competitive manufacturing
The materials used can depend on the battery technology and intended application environment. Advanced batteries like lithium-ion may utilize more complex exotic materials for their terminals and interconnects.
Making Contact with Battery Terminals
For completed batteries, the terminals act as the interface for making electrical contact and extracting current to power devices:
- Small batteries like AA insert into spring loaded clips inside devices.
- Snap or slip-on connectors attach to 9V block battery snaps.
- Coin cell holders touch the flat sides of the battery.
- Wires solder directly to lithium polymer cells.
- Bolts clamp onto lead-acid automotive battery posts.
Proper contact is needed between the terminal and mating connector to keep resistance low. A bad connection can cause power loss, heat generation, arcing and sparks, and excessive voltage drop.
Some tips for making good contact with battery terminals:
- Use connectors sized for the terminal and battery type.
- Ensure surface is clean – clean with alcohol to remove dirt, grease.
- Soldering wires directly provides optimal conductivity.
- For threaded posts, tightly fasten connectors.
- Spring loaded clips should have enough tension for grip.
- Avoid side loading the terminal to reduce strain.
- Apply dielectric grease to prevent corrosion.
Proper battery orientation and polarity matching is also critical as discussed previously. Overall, solid mechanical fit and electrical contact ensures optimal power transfer and battery performance.
Damaged Battery Terminals
Under certain conditions, battery terminals can become corroded or mechanically damaged:
- Sulfation or buildup from electrolyte leakage
- Growth of fuzzy lead-oxide deposits
- White ashy potassium carbonate deposits on alkaline batteries
- Green copper oxide or blue-green copper chloride corrosion
- Rust formation if terminal is uncoated steel
- Bending or breaking from stress or impact
Signs of terminal damage include:
- Visibly corroded, discolored or dirty terminals
- High resistance or variable/intermittent contacts
- Sparking or arcing when connecting loads
- Heat generation at the terminal
- Reduced run time and capacity
- Inability to power devices properly
Some ways to improve battery terminal connections:
- Clean the terminals using a wire brush, sandpaper, or baking soda+water paste.
- Replace severely corroded or damaged terminals if possible.
- Use electrical contact cleaner spray to remove residues.
- Apply dielectric grease to terminals to prevent future corrosion.
- Ensure mating connectors are clean and making proper contact.
- Check for loose connections and broken wires.
Well-maintained clean battery terminals ensure optimal conductivity and battery performance.
Conclusion
In summary, the positive and negative terminals of a battery are referred to as the cathode and anode respectively. The cathode connects to the positive pole and accepts electrons from the external circuit, while the anode supplies electrons through the negative pole. Proper terminal construction, material selection, polarity, and mating connections enable batteries to efficiently deliver power to devices. Keeping the terminals clean helps maintain peak conductivity and prevent damage over the battery lifetime.